CDC authorizes COVID-19 vaccines for children 5-11 years of age
Author

Blaire Bryant
Upcoming Events
Related News

Key Takeaways
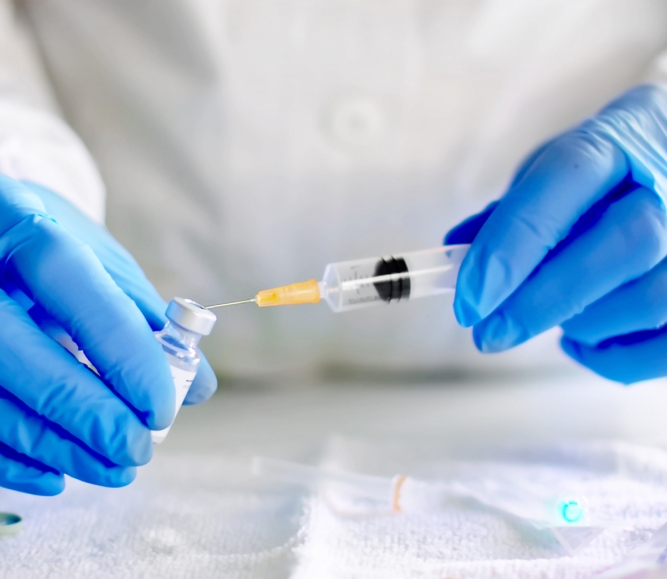
On November 2, the Centers for Disease Control and Prevention (CDC) approved the use of COVID-19 vaccines for children aged 5-11 following the Food and Drug Administration’s (FDA) recommendation. The CDC’s endorsement follows the FDA’s recommendation on October 29 to authorize emergency use of the Pfizer-BioNTech COVID-19 vaccine to include children 5-11 years of age.
The FDA’s decision comes after it was determined the vaccine was approximately 91 percent effective in preventing COVID-19 in children 5-11 years of age. Children within this age group account for 39 percent of COVID-19 cases in individuals younger than 18 years of age across the nation. An ongoing study discovered no serious side effects in approximately 3,100 children aged 5-11 who received the COVID-19 vaccine.
On October 14, the CDC released a Pediatric COVID-19 Vaccination Operational Planning Guide, which highlights key differences between adult and adolescent vaccination programs and those that are designed for children. The National Association of County and City Health Officials (NACCHO) has provided a summary of the guide and pulled out high-level considerations for county health departments, as key components of pediatric COVID-19 vaccination planning in local communities.
As key providers of local public health services and frontline service providers for the medically vulnerable, counties have supported over 192 million vaccinations in the United States to date and will continue to play an essential role in the administration of COVID-19 vaccines and boosters. For more information on how county health facilities can prepare for the distribution of COVID-19 vaccines for children, and COVID-19 boosters, see NACo’s COVID-19 Vaccine Distribution Toolkit.
ADDITIONAL RESOURCES
Resource
COVID-19 Vaccine Toolkit for Counties
Related News

Drug tracking software helps counties identify trends, save lives
Florida counties are using an artificial intelligence tool called Drug TRAC to track and report drug trends, with the aim of providing quicker outreach and saving lives.

White House Executive Order establishes national substance use disorder response
On January 29, the White House issued an Executive Order (EO) establishing the Great American Recovery Initiative, a new federal effort aimed at coordinating a national response to substance use disorder (SUD).

USDA and HHS release new dietary guidelines
On January 7, U.S. Department of Agriculture Secretary Brooke Rollins and U.S. Department of Health and Human Services Secretary Robert F. Kennedy, Jr. unveiled the new Dietary Guidelines for Americans, 2025–2030.