FEMA offers support to combat latest COVID surge
Upcoming Events
Related News

Key Takeaways
Funding under FEMA’s Public Assistance Program continues to support counties for COVID-19 testing, vaccination administration, expanding hospital capacity and surge staffing.
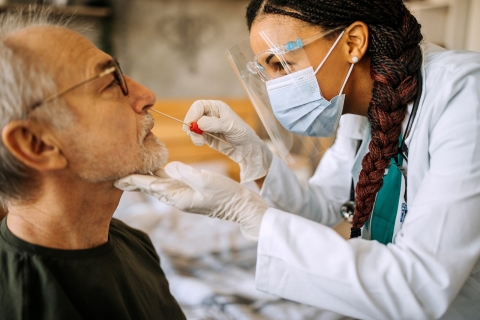
Testing
FEMA will fund testing to detect COVID-19 infections. This includes both in a medical setting (primary medical care facilities, temporary medical care facilities, and community-based testing sites) and testing needed to safely open and operate public facilities, including schools and government offices. Funding may be used to support both diagnostic and screening protocols.
In general, FEMA will fund diagnostic and screening testing to determine if an active coronavirus infection is present and if an individual should take steps to quarantine or isolate from others.
This funding may include costs for:
- Laboratory testing materials and test kits including antigen self-tests.
- Contracting for testing support by a third party.
- Testing facilities, including temporary facilities or expansion of space used for testing.
- Staffing to administer tests.
- Training for individuals to administer tests.
- Signage and other communication materials.
- Personal protective equipment and other administrative supplies to conduct testing and technology to register and track testing results.
- Mobilization and demobilization of testing activities.
- Facility operating costs (equipment, supplies, security, cleaning/disinfection).
Hospital capacity
FEMA will fund medical care to treat people suffering from COVID-19 infections. This includes medical care in primary care facilities such as hospitals, and funding for temporary and expanded medical care capacity. This funding may include costs for:
- Medical care for COVID-19 patients.
- Surge staffing for medical care personnel.
- Expansion of hospitals and medical facilities, such as conversion of unused or other space for clinical care.
- Establishing and operating temporary medical facilities, including lease, purchase, and construction costs.
- Mobilization and demobilization for setting up and closing temporary or expanded medical capacity.
- Operating costs, including equipment, supplies, staffing, and wraparound services.
- Funding may be based on ongoing and projected needs regarding continuing operations at a temporary or expanded medical facility.
- In most cases, permanent renovations are not eligible, except where the work is completed in time to meet COVID-19 capacity needs and is cost-effective. Permanent renovations are also subject to real property disposition requirements.
Vaccinations
FEMA will fund the administration of vaccinations for COVID-19. This includes carrying out vaccination administration for people of any CDC-approved age and boosters. This funding may include costs for:
- Vaccination facilities, including community vaccination centers, mass vaccination sites, and mobile vaccinations, including necessary security and other services for sites.
- Medical and support staff, including contracted and temporary hires to administer vaccinations.
- Training and technical assistance for storing, handling, distributing, and administering of vaccinations.
- PPE, other equipment, supplies, and materials required for storing, handling, distributing/transporting, and administering vaccinations.
- Transportation support, such as refrigerated trucks and transport security, for vaccine distribution as well as reasonable transportation to and from the vaccination sites.
- Onsite infection control measures and emergency medical care for children and families at vaccination sites.
- Communication efforts that keep the public informed, including messaging campaigns, flyers, advertisements, websites, translation services, community engagement, and call centers or websites to assist with appointments or answering questions.
Frequently asked questions
How soon can funding be available?
Organizations should immediately take action to save lives and protect public health and safety. If applicants have an immediate need for funding, FEMA can expedite up to 50 percent of funding needs for medical care and expansion in hospital capacity to eligible applicants with limited documentation required. All entities can apply for FEMA funding through the COVID-19 Public Assistance Simplified Application. Applicants can begin their application by contacting their emergency management office or visiting grantee.fema.gov.
Is there a cost share?
FEMA will fund 100 percent of eligible COVID-19 costs and will not require non-federal matching funds through April 1, 2022.
Can the National Guard assist?
States may continue to utilize National Guard resources under Title 32 authorizations to support COVID-19 medical care, testing and vaccination activities. FEMA will fund 100 percent of National Guard costs through April 1, 2022.
How is FEMA ensuring that testing, medical care and vaccination efforts are equitable?
President Biden’s Executive Order on Ensuring an Equitable Pandemic Response and Recovery requires all states and local governments to focus the use of FEMA funding on the highest-risk communities and underserved populations. State, tribal nation, and territory recipients of FEMA funding are required to ensure that all state, tribal nation, territorial, local, and private non-profit subrecipients are adhering to applicable federal civil rights and non-discrimination laws and equity data requirements. The DHS Civil Rights Evaluation Tool provides an overview of what is required to meet civil rights requirements. Each Recipient’s FEMA-State Agreement includes specific conditions related to these responsibilities.
What if states, tribal nations and territories have outstanding needs for assistance?
Local governments that have outstanding needs or gaps in what they need to address for the ongoing surge in Omicron variant COVID-19 infections should contact their FEMA Regional Office.
How can applicants learn more about FEMA PA Funding?
To learn more about FEMA PA’s program, eligibility, and how to apply for funding, visit fema.gov.
Attachments
Related News

HHS issues termination notices for health grant funding
On March 25, the U.S. Department of Health and Human Services (HHS) sent letters to state authorities and counties with direct grant funding announcing the immediate termination of several pandemic-related grants, which was previously set to run through September 2025.

New funding boosts Indiana county health departments
An infusion of state funding has helped counties support local nonprofits on their way to improving local health outcomes.

HHS announces major restructuring
On March 27, the U.S. Department of Health and Human Services (HHS) announced a sweeping reorganization that will consolidate agencies, shift key programs under a new framework and eliminate thousands of positions. This change brings HHS in line with President Trump's Executive Order, “Implementing the President’s ‘Department of Government Efficiency’ Workforce Optimization Initiative.”